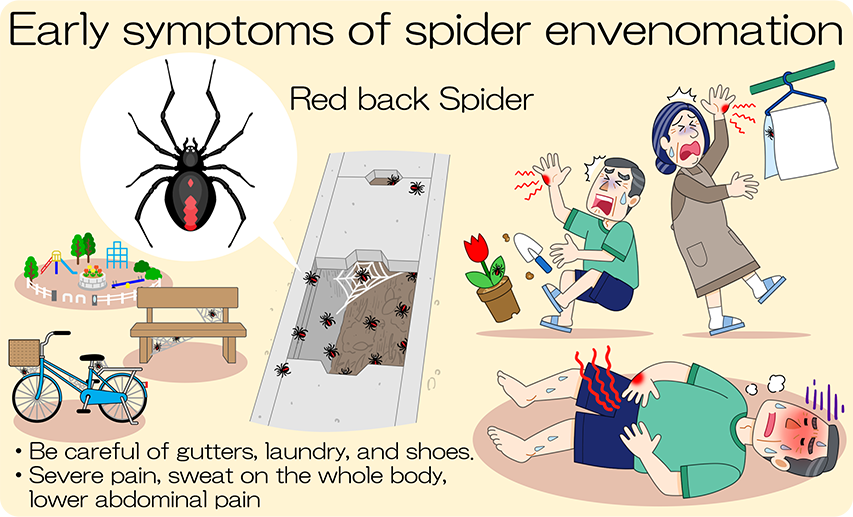
The redback spider is a small, venomous spider that is found only in females. Its Japanese name means “red back spider,” and it is widely distributed from East Asia to Australia and the South Pacific. It was not previously found in Japan, but since its discovery in September 1995 in Takaishi City, Osaka Prefecture, and then in Yokkaichi City, Mie Prefecture, it has expanded its habitat throughout Japan, and as of August 2019, it is a specified alien species distributed throughout Japan except in Akita and Aomori Prefectures. Although it is not aggressive, it inhabits areas close to human living environments, such as inside gutters, gaps in their metal lids, inside drainage pipes in residential areas, at the base of fences, and inside blocks in flower beds. Immediately after a bite, a person may feel only a slight pain, but the pain gradually increases and may cause abdominal or chest pain. Although infrequent, severe cases can cause vomiting, fever, hypertension, tachycardia, and other neurotoxic symptoms.
In Australia, the country of origin, there have been reports of fatal cases, and antivenom treatment is used for severe cases. The number of bite cases in Osaka Prefecture, where domestic survey data are available, has been published at 96 cases from 1995 to 2019, but the number of bites has clearly increased from 2006 to 2013, mainly in Osaka Prefecture, and has leveled off since then.
The expansion of the redback spider habitat in Japan and the increase in bite cases indicate the expansion of bite opportunities for people of all ages. Severe cases of bites are accompanied by systemic symptoms and severe pain. In addition, the exotic toxic organisms have expanded their habitat into the immediate environment, and the stings are very painful and severe cases have caused great concern among the public. The fact that no deaths have been reported in Australia due to redback spider bites since the development of CSL’s antivenom also increases the need for antitoxin products for treatment. In response to this situation, the MHLW and AMED research funded in 2013, “Research on Quality Control of Antivenoms and Therapeutic Methods Using Antivenom”: Toru Hifumi, Principal Investigator (St. Luke’s International University), conducted a clinical study on adverse reactions to the importation of red squirrel spider antitoxin and the use of domestic Yamakagashi antivenom. The study included the development of an assurance system as a countermeasure, the examination of smooth transportation of antivenom in the event of a bite, and the examination of stockpiling sites.
As a countermeasure against red-eyed spider bites, we had planned to purchase a certain number of red-eyed spider antitoxin manufactured by CSL, Australia, for personal import by doctors, and provide it as a treatment for any bite cases. In the course of the research team’s work on each rare disease, there has been a significant delay in the personal import of overseas antivenom products. In 2015, a decision was made to produce a domestic version of the redback spider antivenom. In 2015, a decision was made to produce a domesticated septopus antitoxin for the septopus, which would provide a stable supply and an adequate response to outbreaks.

In order to deal with severe cases of bites by the Japanese redbacked spider, which is a specified invasive species designated by the Ministry of the Environment and has spread its habitat almost nationwide in the 25,6 years since it was first discovered in Japan, a domestic redbacked spider antivenom was produced and GLP-compliant safety tests were completed without any problems. In order to use this domestic antivenom in humans, another requirement was to conduct Phase I clinical trials, and the results of these trials would have allowed the drug to be used in the clinical research of the antivenom team, but Phase I clinical trials have not been conducted. This antitoxin is an unapproved drug, and the study plan was based on the consideration that a safety study of the drug is essential for its administration to humans. However, after three years, the research group has expanded its research activities, including a change in the leader, and there have been discussions about the position of this antitoxin as an approved drug, so no human administration studies have been conducted.
This antivenom has been in production for three years. Unlike foreign antitoxin preparations, the shelf life of the lyophilized form of the domestically manufactured Horse antivenom is specified as 10 years. The quality of the mountain ash antitoxin, one of the objectives of the research team, has not deteriorated significantly in the 20 years since its production. The results of quality control tests conducted three years after the production of the Japanese redback spider antitoxin showed no deterioration in its quality. Although a large amount of domestically produced antitoxin, which is expected to be more potent than foreign products, has been produced, it has not been used at all. It is hoped that these antivemnoms will be made available to the public so that we will not have to depend on foreign products, which are in unstable supply. To date, we have been able to import and stockpile foreign-made antivenom preparations. Fortunately, there have been no serious cases in which the antitoxin has to be administered for treatment. However, the procurement of antivenom preparations from overseas is not stable, and the supply of antitoxin is a tightrope walk, as we have to look for importers whenever the expiration date approaches.
Since the redback spider bite is a remarkably rare disease and clinical trials in humans are virtually impossible, close consultation between the responsible manufacturing company, the PMDA (the approval department), and the Ministry of Health, Labor and Welfare (MHLW) is required before this antitoxin can be published as an approved drug.
Production of domestic redback spider antitoxin and preclinical studies
Department of Safety and Laboratory Medicine, National Institute of Infectious Diseases (NIID)
Blog 2021.07.19 Dr. Akihiko Yamamoto described the details of the production of domestic redback spider antivenom and non-clinical studies.